01.12.2019
Fluoride toothpastes have been used for many years to prevent tooth decay. They are believed to suppress pathogenic bacteria and protect tooth enamel well. However, more and more often you can hear that this substance is poisonous. How likely is it to get seriously ill using such hygiene products? Is it worth buying fluoridated toothpastes or should you avoid store shelves with such products? Let's try to figure it out.
Very active and destructive
What is fluoride? This is the most active chemical substance, a powerful oxidizer, a poisonous gas. Even water burns in it, resulting in the formation of hydrofluoric acid. It is no coincidence that from ancient Greek this word can be translated as “destruction, damage.” It is not for nothing that the substance was assigned the second hazard class. This means that when fluoride comes into contact with the body, damage to the skin and internal organs occurs.
This gas does not occur in nature in its pure form, but is part of fluoride compounds. Soil, plants and organisms mostly contain calcium fluoride, which is poorly soluble in water. Sodium fluoride is also found, which is especially toxic, dissolves well in water and can poison the body. By the way, sodium fluoride was previously used to combat harmful rodents; rat poison was produced on its basis.
Toothpastes most often contain the same sodium fluoride, but in very small quantities. Its use for oral hygiene is precisely based on its ability to kill all living things. It turns out that we are constantly in contact with an extremely toxic substance and don’t even suspect it. The picture is bleak, isn’t it? But you shouldn't make hasty conclusions.
Fluoride is good at suppressing harmful bacteria that undermine dental health and you can’t argue with that. Another thing is that, once inside the body, it can cause harm. The lethal dose of sodium fluoride is from 5 to 10 g, so you need to make sure that children do not play around while brushing their teeth. A child may be harmed if they eat too much toothpaste.
And yet, the properties of fluorides cannot be looked at one-sidedly, because their action is not limited to destruction.
Dangerous enemy or protector of teeth?
We must pay tribute to fluorides - they are required by the body because they are part of the tooth enamel and skeleton. Along with calcium, phosphorus and magnesium, they are necessary for bone growth. However, to replenish their reserves, a person more than has enough fluoride-containing products and drinking water. The daily requirement of an adult for this substance is only 3-4 mg.
A lack of fluoride is harmful to humans, as is its excess - in this case, growth and bone mineralization are impaired, and tooth enamel is destroyed. It is no coincidence that calcium fluoride is found in many organisms. This compound was found in plants: onions, lentils, tomatoes, hazelnuts, green tea. They are also found in ordinary water.
In some regions, the water is oversaturated with fluoride, so people there suffer from a disease such as fluorosis. At the same time, the condition of not only the teeth, but also the bones deteriorates. The main symptom of the disease is yellow or brown spots appearing on the tooth enamel. Treating this pathology is extremely problematic.
The fight against fluoride: complete victory is still far away
Today, fluoride toothpastes are under attack by activists and some dentists. But how did fluoride become the object of criticism, if just yesterday it was considered the main fighter against caries? Here is a brief chronology of events.
The leader of the fight against fluoride was the former head of the department of cellular biochemistry at the National American Cancer Institute, Dean Berg. In 1977, he spoke in the press with accusations against companies using fluoride.
Together with the Water Safety Foundation, Berg conducted a study and came to the conclusion that an excess of this substance in the body provokes cancer. Japanese scientists from the College of Dentistry in Nippon share the same opinion.
Articles appeared in the press accusing the American government of using fluorides to purify water to get rid of fluoride reserves accumulated during the Cold War. Companies producing hygiene products containing sodium fluoride were also ostracized.
In 1991, the U.S. Public Health Service released a report indicating that the average American's fluoride intake exceeded 6.5 mg per day, well above safe levels. The main sources of the substance entering the body: fluoridated water, toothpastes, drinks, food. How does fluoride get into food? The fact is that this is a component of fertilizers that are used in the States.
Under pressure from public organizations, the US government has required manufacturers to place warnings on fluoride toothpastes. In particular, companies must indicate that ingestion of this product can cause poisoning. Since 1997, such a warning has been included on all tubes of toothpaste containing fluoride. Also, as a result of the publication of these facts, many countries have abandoned water fluoridation.
However, all this hype in the press for corporations producing toothpastes means little so far. If we compare the anti-fluoride fight with a war, the enemy is defeated, but has not yet laid down his arms.
The perfumery and cosmetics industry is not going to give up such a profitable business. In addition, scientific research is being conducted in order to obtain a compound that will be completely safe. Today, tin fluorides are increasingly used in hygiene products, which are supposedly less dangerous compared to sodium fluoride.
Fluoride toothpaste
The most commonly used drug that contains fluoride in its composition is fluoride toothpaste. There are several tips to help maximize the positive effects of fluoride:
— toothpaste should be in the oral cavity for at least 2-3 minutes (time of brushing teeth);
— frequency of brushing teeth – 2 times a day;
- after brushing, you should rinse your teeth with water only once, “filtering” it between your teeth;
— at night, it is recommended to fill the interdental spaces with fluoride toothpaste, applying it to the dental floss.
Well, we talked about the benefits of fluoride for teeth. This is a scientifically substantiated, proven fact. But why then are toothpastes with the proud inscription “Fluoride Free” increasingly conquering the market? Perhaps fluoride is still harmful to teeth?
Why is excess fluoride harmful?
Experiments were conducted abroad in which the effect of fluorides on rodents was studied. It turned out that a high dose of these substances increases the number of malignant tumors, reduces attention and intelligence. In some animal studies, fluoride reduced the number of offspring produced because the levels of female sex hormones dropped.
The danger posed by fluoride toothpastes is that children often swallow their contents. And the reason for this is not pampering, but the increased swallowing reflex of babies. Scientists have identified the consequences of excess fluoride in the body:
- fluorosis;
- the condition of bone tissue deteriorates;
- arthritis develops;
- the thyroid gland suffers;
- the functioning of the endocrine system is disrupted;
- early puberty occurs in girls;
- Reproductive function decreases;
- the likelihood of cardiovascular diseases increases;
- intelligence and attention decline.
In addition, if you receive fluoride in excess, calcification of the ligaments begins. But as a rule, it is not the toothpaste that is to blame for such “joys”. Most often, workers in the chemical industry, as well as those who drink fluoridated water, suffer from excess fluoride in the body.
The insidiousness of fluorides is that they act slowly, accumulating in the body for years, gradually eroding health. To reduce the harmful effect, it is recommended to use a volume of toothpaste not exceeding a pea for one brushing of teeth. And it is better not to use hygiene products with fluorides at all. You can read more about the harm caused by fluoride compounds here.
The effect of fluoride on teeth
What does fluorine have to do with it? The answer is simple: the effect of fluoride on teeth is realized precisely through these processes.
Fluorine, or more correctly fluoride, can be stable or labile.
Stable fluoride is one that is included in the hydroxyapatite crystal lattice instead of the OH group. This happens both before the tooth erupts and after. A new compound is formed - fluorapatite. Fluorapatite is more stable, resistant to acid (its dissolution begins at pH = 4.5), less soluble (it precipitates more easily than hydroxyapatite).
Everything would be fine, but in the conditions of the oral cavity, fluoride in the crystal lattice of enamel does not change its properties so much. The fact is that the enamel contains many crystals with inclusions of CO3 groups - biological carbonapatites. The effect of fluorine on carbonapatite is not as pronounced.
Therefore, today it is believed that the main protective mission is carried out by another fluoride - labile . This is fluoride that is found near the surface of the enamel. He has several sources:
— dissolving stable fluoride;
- dissolving calcium fluoride. It is formed simultaneously with fluorapatite if there is a lot of fluorine for a long time in an acidified environment (there are more calcium ions);
- from intercrystalline enamel fluid;
- from dental plaque and oral fluid.
Labile fluoride affects:
- Demineralization;
- Remineralization, including during initial caries, stabilizing it;
- Activity of dental plaque.
Let's take a closer look at each of the points:
- Labile fluoride prevents demineralization. It maintains the right amount of fluoride ions, and the enamel fluorapatite does not need to dissolve to replenish this amount.
- Labile fluoride helps remineralization. This happens due to several effects of fluoride:
Firstly, the precipitation of calcium and phosphate ions is facilitated. It creates this opportunity under much less favorable conditions (more acidic environment and fewer ions). Fluoride binds them, forming fluorapatite and calcium fluoride. When deposited, these minerals, on the one hand, block the entrances for acid into the enamel, and on the other hand, they interfere with the removal of calcium and phosphates from the enamel. It is also a good supply of ions for the inner layers of the crystal lattice.
Secondly, fluoride accelerates the growth of enamel apatite crystals.
Thirdly, fluorine penetrates more easily and quickly between enamel crystals in the composition of HF acid. It is then released from the acid and repairs and strengthens the crystals themselves.
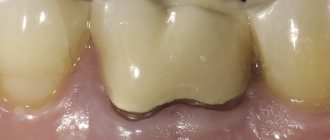
There is one interesting point in the effect of fluoride on a carious lesion. It is because of it that a lesion characteristic of caries is formed with a harder surface layer (1) and a demineralized subsurface (2) even at very low concentrations. There are many opinions about why this happens. In any case, a dentist in a clinic should always remember this feature when examining teeth and diagnosing caries.
- Labile fluoride affects the activity of dental plaque.
In dental plaque, fluoride can be found both outside and inside the microbial cell. As the plaque environment becomes more acidic, more HF acid is formed. This acid easily penetrates the microbial cell. The H+ ion acidifies its cytoplasm, and the F– ion has the following effect:
- Reduces the activity of enolase, an enzyme in the glycolysis reaction, the final product of which is destructive lactic acid;
- Reduces ATP formation. And this is the “key” of cellular metabolism, growth, and a necessary element for the operation of the enzyme that transports glucose into the cell - a source for acid production, and the H + /ATPase enzyme, which maintains the necessary pH inside the cell. Plus, thanks to the work of this same H+/ATPase, the energy that is needed for the life of the microorganism is generated. The enzyme does not work - there is no energy for the cell.
- Fluoride inhibits the microbial cell's synthesis of the macromolecules it needs - proteins, carbohydrates, including extracellular ones - levans, glucans. Levan and glucan are produced by Str. mutans to organize, unite dental plaque (to make it easier for other bacteria to attach and live on the tooth).
If you go beyond the microbial cell, fluoride reduces the growth rate of dental plaque and reduces the content of Str.mutans - the main culprit of caries. It is more sensitive to fluoride than other microorganisms, and the pH of dental plaque in the presence of fluoride is not low enough for it to live comfortably and produce acid.
But outside the laboratory, in vivo, the effects of fluoride on dental plaque are controversial:
- A lot of fluoride is needed to achieve a bactericidal concentration;
- Microorganisms adapt to dangerous levels of fluoride.
Fluoride has a more pronounced effect directly on dental plaque due to other ions with which it is combined in preventive preparations.
Echoes of the arms race
Until the mid-20th century, only specialists knew about fluoride. Everything changed in the 1940s, when the United States turned to it during the development of atomic weapons - the chemical was needed to process uranium ores. Then they tried to use it as a component of rocket fuel, but abandoned this idea due to the high toxicity of the substance.
Fluorides are still used in industry today, so industrial emissions contain these substances. These are components of freons, which today are considered the main destroyers of the ozone layer. Fluorides are present in some medications; for example, fluorouracil is a well-known antitumor agent.
Few people know that fluorine is used to produce Teflon-coated cookware. The real name of Teflon speaks for itself - polytetrafluoroethylene. This material is also called fluoroplastic.
Fluorides are still used in the United States for antibacterial water purification. This method began to be used in the 1940s, when there was an urgent need to find a means for disinfection and there was little concern for safety. In countries such as Finland, Canada, and Israel, water fluoridation was abandoned.
Which toothpastes are better?
The question arises: “If fluorides are so dangerous, wouldn’t the solution be to abandon them altogether?” Dentists do not have a clear opinion on this matter. On the one hand, fluoride compounds are toxic, and on the other, they protect tooth enamel. However, given their ability to accumulate in the body, it is better to opt for products that do not contain fluoride.
You also need to take into account that toothpastes are filled with other, no less dangerous chemicals. Among the main anti-heroes is sodium lauryl sulfate, which manufacturers designate with the abbreviations SLS and SLES. This substance was previously used to wash engines, and is now a component of hygiene products. It is contained not only in toothpastes, but also in shampoos, shower gels, and shaving foams.
Scientists from Oslo believe that SLS provokes ulcerative lesions of the oral cavity, causing a disease such as aphthous stomatitis. They also suggest that this component dries out the oral mucosa.
If you want to protect yourself from the harmful effects of fluoride, then today there are every opportunity for this. The market is replete with toothpastes that do not contain this “suspicious subject” at all. They contain other active ingredients to destroy harmful bacteria, such as zinc. It slows down the formation of plaque and tartar well.
Calcium compounds are also used as an alternative to fluorides: calcium citrate, calcium pantothenate, calcium lactate, calcium glycerophosphate, synthetic hydroxyapatite. When the composition contains such compounds, you will not find fluorides there. Also, natural ingredients are included in toothpastes, for example, calendula, eleutherococcus. This is understandable, since calendula is one of the most powerful plants in terms of anti-inflammatory effects. Here is just a short list of fluoride-free toothpastes: PresidentUnique, Rocs, Biocalcium, Maximum, Asepta.
How to strengthen teeth effectively and without harm to health?
In the prevention of tooth decay and gum disease, hygiene is of paramount importance. At the same time, it is not only toothpastes that protect against pathogenic bacteria. The main thing to remember is that microbes multiply when the acid-base balance is disturbed. And you can restore this balance if you simply rinse your mouth thoroughly after eating.
Diet is also very important. Don't forget to eat solid plant foods: carrots, apples, cabbage. This will strengthen your teeth and gums and help cleanse your mouth naturally.
To prevent periodontitis, it is recommended to take a supplement such as Osteomed. It contains calcium citrate, which is perfectly absorbed, and another component of the drug is drone homogenate. It is the source of all the amino acids, vitamins and minerals needed for our health. Calcium citrate supplies the mineral to teeth and bone tissue, and drone homogenate normalizes hormonal levels and improves metabolic processes. The action of these two components gives a synergistic effect, which perfectly protects teeth and gums from diseases.
Category Hygiene Published by Mister stomatolog
Sodium fluoride 2.2 mg 250 pcs. lozenges
pharmachologic effect
Fluoride drug.
Composition and release form Sodium fluoride 2.2 mg 250 pcs. lozenges
Tablets - 1 tablet:
- active substance - sodium fluoride 2.2 mg;
- excipients: lactose monohydrate, calcium stearate, potato starch.
Lozenges for children 2.2 mg.
10 tablets each in a blister pack made of polyvinyl chloride film and aluminum foil. 3 blister packs along with instructions for use in a cardboard pack.
250 tablets in a plastic bottle. 1 bottle along with instructions for use in a pack.
Description of the dosage form
Round flat-cylindrical tablets of white color with a chamfer.
Directions for use and doses
Children aged 2 to 6 years - 1 tablet (1.1 mg) 1 time per day.
Children over 6 years old - 1 tablet (2.2 mg) or 2 tablets (1.1 mg) 1 time per day. It is recommended to take the drug before bed after brushing your teeth. The tablet is kept in the mouth until completely dissolved. The duration of treatment is at least 250 days a year, annually until the age of 15. It is not recommended to use medications containing calcium at the same time.
Pharmacodynamics
Sodium fluoride is an anti-caries preventive agent (especially in children and adolescents). Fluoride ions directly affect the processes of mineralization of hard dental tissues during their development. Additional introduction of fluorine ensures the formation of the most stable form of apatite - fluorapatite - in dental tissues. In addition, fluoride helps reduce the cariogenic activity of dental plaque. Inhibits the formation of lactic acid from carbohydrates.
Pharmacokinetics
Absorption when taken orally is 93-97%. TCmax -4 hours. At any dose, 50% of the incoming fluorine accumulates in the hard tissues of the tooth and bone tissue. There is no accumulation in organs or soft tissues. Excreted by the kidneys (fluoride, which is not involved in the formation of bone tissue).
Indications for use Sodium fluoride 2.2 mg 250 pcs. lozenges
Prevention of dental caries in children aged 2 to 14 years (if the concentration of fluoride in drinking water does not exceed 0.7 mg/l).
Contraindications
Hypersensitivity, hypothyroidism, peptic ulcer of the stomach and duodenum (in the acute stage), liver and/or kidney failure.
Pregnancy, lactation period.
Children under 2 years of age.
Living in an area with sufficient fluoride content in water (above 0.7 mg/ml). Sodium fluoride lozenges for children, orange 1.1 mg, contain aspartame, a source of phenylalanine. This drug should not be taken by patients with phenylketonuria.
Sodium fluoride lozenges for children 2.2 mg contain lactose. This drug should not be taken by patients with rare congenital galactose intolerance, lactase deficiency or glucose-galactose malabsorption.
Application Sodium fluoride 2.2 mg 250 pcs. lozenges during pregnancy and breastfeeding
Contraindicated during pregnancy, breastfeeding (abortion of breastfeeding is required during use), children under 6 months, 3 years, 6 or 14 years (depending on the dosage form and dose).
special instructions
Do not exceed recommended doses and follow your dentist's instructions.
Make sure your child does not receive additional fluoride while taking other medications or dietary supplements.
Use in children under 6 years of age requires special monitoring.
To avoid the development of fluorosis, it is advisable to regularly conduct a dental examination of the child’s teeth at least 1-2 times a year.
Overdose
Symptoms: lacrimation, hypersalivation, nausea, loss of appetite, vomiting, diarrhea, abdominal pain, pain in the lower extremities, arthralgia, fatigue, miosis, blurred vision, weakness, myasthenia gravis, tremor, convulsions, increased body temperature, tachycardia, decreased blood pressure pressure, respiratory failure, respiratory arrest.
Treatment: administration of large quantities of fluid and calcium (calcium gluconate or calcium lactate solution, milk) to precipitate fluorides; gastric lavage with acidified water or 1% sodium chloride solution, administration of saline laxatives, intravenous administration of electrolytes (20 ml of 10-20% calcium gluconate solution), vitamins; symptomatic therapy, monitoring calcium concentration in the blood; hemodialysis.
Side effects Sodium fluoride 2.2 mg 250 pcs. lozenges
Nausea, vomiting, loss of appetite, increased fatigue, headache, diarrhea, ossification of the attachment sites of tendons and ligaments, hypothyroidism (with prolonged use), allergic reactions, rhinitis, eosinophilia, skin rash, fluorosis (disruption of the process of formation and calcification of enamel, appearance of yellow , brown spots, mottled spots, increased fragility and wear of teeth).
Drug interactions
Antacids reduce the absorption of sodium fluoride (prematurely dissolve the membrane); It is recommended to use antacids no earlier than 2 hours before taking sodium fluoride.
Vitamins A and D promote the development of ectopic calcification.