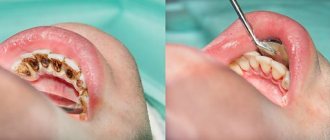
Rotary dental instruments include burs, cutters, discs, abrasive heads, and polishers used in dental practice for processing hard and, in some cases, soft tissues of the maxillofacial area.
Regardless of the functional purpose of rotating dental products, their design includes a so-called working part, with the help of which the material being processed is removed, and a shank, designed to secure the tool in the dental handpiece. The surface of the working part can be made of various materials, for example, steel, diamond chips, corundum, silicone, polymers, natural pile, artificial bristles. These circumstances must be taken into account when choosing methods and means of processing products before subsequent use.
In accordance with the provisions of Ch. V SanPiN 2.1.3.2630-10 “Sanitary and epidemiological requirements for organizations engaged in medical activities” all dental instruments that come into contact with the wound surface, blood or injectable drugs, as well as those that come into contact with the mucous membrane during operation and can cause its damage, must consistently undergo disinfection, pre-sterilization cleaning and sterilization.
Step 1: Disinfection of rotary dental instruments
Purpose: to destroy pathogenic and opportunistic microorganisms that contaminated products during medical procedures.
Disinfection of rotary dental instruments should be carried out immediately after their use, without allowing contaminants to dry on them, by completely immersing them in a disinfectant solution. The thickness of the mortar layer above the products should be at least 1 cm.
Working solutions for disinfecting instruments are prepared in advance. To do this, the required amount of concentrated disinfectant is mixed with water in the proportions specified in the instructions for use of the disinfectant.
For your information
To accurately dose the concentrate, you can use a graduated measuring cup or a disposable injection syringe.
All containers with working solutions of disinfectants must be equipped with tight-fitting lids, have clear inscriptions or labels indicating the product used, its concentration, purpose, preparation date, and expiration date of the solution.
To process dental instruments, special small-volume containers are used, which can significantly reduce the consumption of disinfectants.
Note!
To disinfect medical devices used in dentistry, it is necessary to use disinfectants that have a wide range of antimicrobial activity, including bactericidal, virucidal, and fungicidal properties.
The choice of modes is carried out between viruses or fungi of the genus Candida.
In tuberculosis medical organizations, regimens should be used in which disinfectant solutions are active against mycobacterium tuberculosis.
It is not recommended to use preparations containing aldehydes, since they are capable of fixing organic contaminants on the surface of instruments.
When processing steel and carbide burs, you should avoid using products containing hydrochloric acid and hydrogen peroxide - they can significantly deteriorate the properties of the tools.
Prolonged exposure of carbide and steel burs in the same container with a disinfectant solution can lead to their surface corrosion.
Endoscope leak test
Immediately after preliminary cleaning or immediately before final cleaning (in the final cleaning room), a leak test of the endoscope is performed.
It is strictly forbidden to skip this stage! Data on the tightness of the endoscope is extremely important. It is strictly forbidden to transfer a leaky endoscope at the stage of final pre-sterilization cleaning, especially during HLD or sterilization. Such devices are immediately transferred to the service department for diagnostics and repair. We will tell you more about leak testing and the leak detectors used for this in a separate article on our blog.
Stage 2. Pre-sterilization cleaning of rotary dental instruments
Purpose: to remove protein, fat, mechanical contaminants, residues of medications, filling materials from the surface of instruments, to reduce general microbial contamination to facilitate subsequent sterilization.
Pre-sterilization cleaning (PSC) of products is performed manually or mechanized .
In the manual PSO method, instruments are washed in solutions intended for these purposes. To clean the outer surface of products, use brushes or gauze wipes.
To increase the efficiency of pre-sterilization treatment of rotary dental instruments, especially those that are sensitive to mechanical stress, special ultrasonic washers (baths) are used. Under the influence of ultrasound, water vibrates at a supersonic frequency, affecting all areas of the instrument, including hard-to-reach ones, like a “microscopic brush,” destroying the surface layer of contaminants, which facilitates further processing.
After disinfection and PSO, rotary dental instruments are rinsed under running water for the time recommended in the instructions for the use of disinfectants and detergents, then desalted in distilled water, dried and only then sterilized.
The quality of the performed PSO is assessed by performing an azopyram (or amidopyrine) test for the presence of residual amounts of blood, as well as a phenolphthalein test for the presence of residual amounts of alkaline components (in cases of using detergents whose working solutions have a pH of more than 8.5). The control results are recorded in special journals.
Note!
The most convenient to use are disinfectants that allow you to combine the processes of disinfection and pre-sterilization cleaning of rotating products in one stage.
Ultrasonic units can use disinfectants that have cleaning properties. The advantage of many of them is that they are available in the form of ready-to-use solutions and do not require dilution.
Enzyme-based cleansers can be used for PSO. Enzyme-containing preparations destroy the bonds between the cells of the substrate (biological fluid) and break down biological films on the surface of instruments.
An example of calculating the need for disinfectants for processing instruments is presented below.
Endoscope processing algorithm
Let us first consider the general algorithm for processing an endoscope.
According to the methodological instructions MU 3.5.1937-04, the general sequence of actions when processing and disinfecting endoscopes and instruments for them is as follows:
- Pre-cleaning
- Final cleaning (cleaning combined with disinfection) or pre-sterilization cleaning
- High Level Disinfection (HLD) or Sterilization
- Storage
It is important to fully follow this algorithm; all instructions from health care facilities must be drawn up taking it into account.
|
|
| Pre-cleaning | Pre-cleaning |
| Final cleaning | Pre-sterilization cleaning |
| High level disinfection | Sterilization |
| Aseptic storage | Sterile storage |
Aspirator containers and other peripherals are also subject to disinfection/sterilization.
Step 3: Sterilization of rotary dental instruments
Goal: completely free instruments from pathogenic and non-pathogenic microorganisms, including their spore forms.
When choosing a sterilization method, you should take into account the recommendations of instrument manufacturers. Most manufacturers do not recommend using modes in which temperatures exceed 160 °C, since this can lead to disruption of the structure of the abrasive layer of rotating products.
Note!
It is not advisable to use air and glasperlene methods to sterilize rotary dental instruments. The optimal method is steam sterilization.
Before sterilization, instruments are packaged in special sterilization disposable materials. Sterilized packaged products are stored in cabinets or on work tables. Shelf life is determined by the type of packaging material, as well as instructions for its use.
Sterilization of unpackaged rotary dental instruments is only permitted with a decentralized processing system in the case of portable sterilizers.
Important!
Products sterilized in unpackaged form must be used immediately for their intended purpose.
It is allowed to store sterile instruments in special bactericidal chambers for the period specified in the equipment operating manual, and in the absence of such chambers - on a sterile table for no more than 6 hours.
Sterile products are placed on the doctor’s dental table in a sterile tray or on a sterile napkin immediately before manipulations on a particular patient.
Sterilization control is carried out at each cycle using special chemical test indicators placed at control points.
Modern chemical test indicators allow you to simultaneously evaluate 2 or more critical sterilization parameters and make a conclusion about the suitability of a sterile batch of products for use.
The results are reflected in the sterilizer operation control logs.
Note!
If there are more than three dental chairs in a dental medical organization, PSO and sterilization of instruments are carried out in specially designated sterilization rooms, divided into “clean” and “dirty” zones.
General provisions for reprocessing and disinfection of flexible endoscopy
Flexible endoscopes are divided into devices for non-sterile and sterile endoscopic interventions.
Non-sterile interventions
— endoscopic research operations in which an endoscope is inserted into organs that normally contain their own microflora through natural pathways (gastrointestinal tract, pulmonary system: upper and lower and respiratory tract, outer and middle ear).
Endoscopes for non-sterile interventions:
- Gastroscope
- Colonoscope
- Duodenoscope
- Bronchoscope
- Choledochoscope
In other words, it is necessary to work with gastroscopes, colonoscopes and bronchoscopes in accordance with the rules and algorithms for processing endoscopes for non-sterile interventions.
Sterile interventions:
The endoscope is inserted through punctures and incisions in the skin and mucous membranes into vessels, cavities, tissues, and into normally sterile organs (uterus, bladder) through natural pathways.
Endoscopes for sterile procedures
- Cystoscope
- Urethroscope
- Hysteroscope
- Resectoscope
- Arthroscope
and others. Here we note that the first 4 endoscopes can be produced either rigid or flexible, depending on the purpose of the study or operation.
Devices, installations and apparatus for pre-cleaning the endoscope
Devices for automatic pre-cleaning most often mean and mean devices for cleaning canals. We wrote more about them in the material about installations for washing endoscopes.
Here we note that they can be used both at the stage of preliminary cleaning (in the procedural or operating room), and during final / pre-sterilization cleaning in the washing and disinfection department.
Important! The presence of a device for cleaning channels does not eliminate the need to clean them with special brushes to remove large particles.
The devices have a built-in pump/aspirator for automatic supply of air and disinfectant solution into the endoscope channels. Accordingly, it is necessary to connect all available channels to the device (regardless of whether the doctor used them during the intervention or not). All devices also have a control and monitoring unit. Most often, the time and number of channel cleaning cycles are set using the panel keys. Sometimes programs can be entered and stored in the device's memory.
Let us list the devices for cleaning and washing channels that are popular on our market.
- Scope Buddy (Medivators, Cantel - USA)
- Epw 100 / EPW 100 S (Steelco - USA, Italy)
- E-Clean (Endo Stars - Russia)
- EndoDez (Kront - Russia)
Also, all devices for automatic channel washing have a built-in leak test system, that is, in this case you do not need a separate leak detector (but as a backup option, as well as for checks, its presence is still highly recommended).