Composition and properties of tooth enamel
The composition of tooth enamel consists mainly of inorganic substances (more than 95%). Organic substances are represented by proteins, lipids and carbohydrates. The hardness of the enamel layer is determined by the increased content of inorganic substances in it. About 3% of the composition is water. This is where such high strength is achieved. The chemical composition of enamel is unique. The high content of inorganic substances in it ensures that it performs its main functions - protecting the deeper layers of the tooth. Inorganic substances include hydroxyapatite crystals. It is a mineral that contains various elements, including fluorine, magnesium and others. It is because of these crystals that the enamel is so sensitive to the effects of acids. The integrity and density of the enamel layer depend on calcium ions. Calcium is also part of the enamel of teeth and bones. Enamel, consisting of many elements, has a rich composition, which determines its properties.
Biochemistry of the oral cavity
Biochemistry of hard dental tissues
Such tissues include enamel, dentin, and cementum. These tissues differ from each other by their different origins in ontogenesis. Therefore, they differ in chemical structure and composition. And also by the nature of metabolism. In them, the enamel is of eptodermal origin, and the bone, cement, dentin are of mesentimal origin, but despite this, all these tissues have much in common, they consist of an intercellular substance or matrix of carbohydrate-protein nature and a large amount of minerals, mainly , represented by apatite crystals.
Degree of mineralization:
Enamel -> dentin -> cement -> bone.
These tissues contain the following percentages:
Minerals: Enamel-95%; Dentin-70%; Cement-50%; Bone-45%
Organic substances: Enamel-1 – 1.5%; Dentin-20%; Cement-27%; Bone-30%
Water: Enamel-30%; Dentin-4%; Cement-13%; Bone-25%.
These crystals have a hexogenal shape.
Mineral components of enamel
They are presented in the form of compounds having a crystal lattice
A(BO)K
A = Ca, Ba, cadmium, strontium
B = PO, Si, As, CO.
K = OH, Br, J, Cl.
1) hydroxyapatite - Ca (PO) (OH) in tooth enamel 75% HAP - the most common in mineralized tissues
2) carbonate apatite - CAP - 19% Ca (PO) CO - soft, easily soluble in weak acids, whole, easily destroyed
3) chlorapatite Ca (PO) Cl 4.4% soft
4) strontium apatite (SAP) Ca Sr (PO) - 0.9% is not common in mineral tissues and is common in inanimate nature.
Min. ingredients 1 – 2% in non-apatite form, in the form of calcium phosphate, dicalciferate, orthocalciphosphate. The Ca/P ratio of 1.67 corresponds to the ideal ratio, but Ca ions can be replaced by chemical elements Ba, Cr, and Mg that are similar in properties. At the same time, the ratio of Ca to P decreases, it decreases to 1.33%, the properties of this apatite change, and the resistance of the enamel to adverse conditions decreases. As a result of the replacement of hydroxyl groups with fluorine, fluorapatite is formed, which is superior in both strength and acid resistance to HAP.
Ca (PO) (OH) + F = Ca (PO) FOH hydroxyfluorapatite
Ca(PO)(OH) + 2F = Ca(PO)F fluorapatite
Ca (PO) (OH) + 20F = 10CaF + 6PO + 2OH Ca fluoride.
CaF is durable, hard, and easily leached. If the pH shifts to the alkaline side, tooth enamel destruction, enamel mottling, and fluorosis occurs.
Strontium apatite - in the bones and teeth of animals and people living in regions with a high content of radioactive strontium, they have increased fragility. Bones and teeth become brittle, strontium rickets develops, causeless, multiple bone fractures. Unlike ordinary rickets, strontium rickets is not treated with vitamin D.
Features of the crystal structure
The most typical is the hexogenal form of HAp, but there may be rod-shaped, acicular, or diamond-shaped crystals. All of them are ordered, of a certain shape, have ordered enamel prisms - this is the structural unit of enamel.
4 structures:
a crystal consists of elementary units or cells; there can be up to 2 thousand such cells. Mol.mass = 1000. A cell is a 1st order structure, the crystal itself has Mr = 2,000,000, it has 2,000 cells. Crystal is a 2nd order structure.
Enamel prisms are a 3rd order structure. In turn, enamel prisms are collected in bundles, this is a 4th order structure, around each crystal there is a hydration shell, any penetration of substances onto the surface or inside the crystal is bound in this hydration shell.
It is a layer of water associated with a crystal in which ion exchange occurs, it ensures the constancy of the enamel composition, called enamel lymph.
Water is intracrystalline; the physiological properties of enamel and some chemical properties, solubility, and permeability depend on it.
Type: water bound to enamel proteins. In the structure of HAP, the Ca/P ratio is 1.67. But there are HAPs in which this ratio ranges from 1.33 to 2.
Ca ions in HAP can be replaced by other chemical elements similar in properties to Ca. These are Ba, Mg, Sr, less often Na, K, Mg, Zn, HO ion. Such substitutions are called isomorphic, as a result the Ca / P ratio decreases. Thus, it is formed from HAP - HFA.
Phosphates can be replaced by the PO ion HPO citrate.
Hydroxites are replaced by Cl, Br, F, J.
Such isomorphic substitutions lead to changes in the properties of apatites - the resistance of enamel to acids and to caries decreases.
There are other reasons for changes in the composition of HAP, the presence of vacant places in the crystal lattice, which must be replaced with one of the ions, vacant places arise most often under the action of acids, already in the formed HAP crystal, the formation of vacant places leads to a change in the strength of the enamel, permeability , solubility, adsorptive properties.
The balance between the process of de- and remineralization is disrupted. Optimal conditions for chemicals arise. reactions on the enamel surface.
Physicochemical properties of apatite crystal
One of the most important properties of a crystal is charge. If there are 10 remaining Ca in a HAP crystal, then consider 2 x 10 = 3 x 6 + 1 x 2 = 20 + 20 = 0.
HAP is electrically neutral; if the HAP structure contains 8 Ca – Ca (PO) ions, then 2 x 8 20 = 16 < 20, the crystal acquires a negative charge. It can also be positively charged. Such crystals become unstable. They are reactive and surface electrochemical imbalance occurs. ions are in a hydration shell. They can neutralize the charge on the surface of apatite and such a crystal again becomes stable.
Stages of penetration of substances into a HAP crystal
3 stages
1) ion exchange between the solution that washes the crystal - this is saliva and dental fluid with its hydration shell. It receives ions that neutralize the charge of the crystal: Ca, Sr, Co, PO, and citrate. Some ions can accumulate and also easily leave without penetrating into the crystal - these are K and Cl ions, other ions penetrate into the surface layer of the crystal - these are Na and F ions. The stage occurs quickly within a few minutes.
2) this is an ion exchange between the hydration shell and the surface of the crystal; an ion is separated from the crystal surface and replaced with other ions from the hydration shell. As a result, the surface charge of the crystal is reduced or neutralized and it becomes stable. Longer than stage 1. During few hours. Ca, F, Co, Sr, Na, P penetrate.
3) Penetration of ions from the surface into the crystal - called intracrystalline exchange, occurs very slowly and as the ion penetrates, the speed of this stage slows down. The ions Pa, F, Ca, Sr have this ability.
The presence of vacancies in the crystal lattice is an important factor in the activation of isomorphic substitutions within the crystal. The penetration of ions into the crystal depends on the R ion and the level of E it possesses; therefore, H ions, which are similar in structure to the H ion, penetrate more easily. The stage lasts for days, weeks, months. The composition of the HAp crystal and their properties are constantly changing and depend on the ionic composition of the liquid that washes the crystal and the composition of the hydration shell. These holy crystals make it possible to purposefully change the composition of hard tooth tissues under the influence of remineralizing solutions for the purpose of preventing or treating caries.
Organic substances of enamel
The share of organic matter 1 is 1.5%. In immature enamel up to 20%. Organic substances of enamel influence the biochemical and physical processes occurring in tooth enamel. Org.v-va nah-xia between apatite crystals in the form of bundles, plates or spirals. The main representatives are proteins, carbohydrates, lipids, nitrogen-containing substances (urea, peptides, cyclic AMP, cyclic amino acids).
Proteins and carbohydrates are part of the organic matrix. All remineralization processes occur on the basis of a protein matrix. Most of it is represented by collagen proteins. They have the ability to initiate remineralization.
1. a) enamel proteins – insoluble in acids, 0.9% EDTA. They belong to collagen- and ceramide-like proteins with a large amount of sulfur, hydroxyproline, gly, and lys. These proteins play a protective role in the process of demineralization. It is no coincidence that in the focus of demineralization at the stage of a white or pigmented spot, the number of these proteins is > 4 times. Therefore, a carious spot does not turn into a carious cavity for several years, and sometimes caries does not develop at all. In older people, caries > resistance. b) calcium-binding proteins of enamel. KSBE. They contain Ca ions in a neutral and slightly alkaline environment and promote the penetration of Ca from saliva into the tooth and back. Proteins A and B account for 0.9% of the total mass of enamel.
2. B. soluble in water, not associated with mineral substances. They do not have an affinity for the mineral components of enamel and cannot form complexes. There are 0.3% of such proteins.
3. Free peptides and individual amino acids, such as promine, gly, val, hydroxyproline, ser. Up to 0.1%
1) protective function. Proteins surround the crystal. Prevents the process of demineralization
2) proteins initiate mineralization. Actively participate in this process
3) provide mineral exchange in enamel and other hard tissues of the tooth.
Carbohydrates are represented by polysaccharides: glucose, galactose, fructose, glycogen. Disaccharides are in free form, and protein complexes are formed - phospho-glycoproteins.
There are very few lipids. Presented in the form of glycophospholipids. During the formation of the matrix, they act as connecting bridges between proteins and minerals.
Dentin is inferior in hardness. The most important elements of dentin are the ions Ca, PO, Co, Mg, F. Mg is 3 times more than in enamel. The concentration of Na and Cl increases in the inner layers of dentin.
The main substance of dentin consists of HAP. But unlike enamel, dentin is penetrated by a large number of dentinal tubules. Pain sensations are transmitted through nerve receptors. The dentinal tubules contain processes of odontoblast cells, pulp and dentinal fluid. Dentin makes up the bulk of the tooth, but is less mineralized than enamel; its structure resembles coarse-fibered bone, but is harder.
Organic matter
Proteins, lipids, carbohydrates, ...
Protein matrix of dentin - 20% of the total mass of dentin. Consists of collagen, it accounts for 35% of all organic dentin. This property is characteristic of lysine tissues of normal origin; it contains glucosaminoglycogens, galactose, hexasamites and heliuronic acids. Dentin is rich in active regulatory proteins that regulate the remineralization process. Such special proteins include amelogenins, enamelins, and phosphoproteins. Dentin, like enamel, is characterized by a slow exchange of minimal components, which is of great importance for maintaining tissue stability under conditions of increased risk of demineralization and stress.
Tooth cement
Covers the entire tooth with a thin layer. Primary cement is formed by a mineral substance in which collagen fibers and cellular elements - cementoblasts - pass in different directions. The cement of a mature tooth is little renewed. Composition: mineral components are mainly represented by Ca carbonates and phosphates. Cementum, like enamel and dentin, does not have its own blood vessels. In the apex of the tooth there is cellular cement, the main part is acellular cement. Cellular resembles bone, and acellular consists of collated fibers and an amorphous substance that glues these fibers together.
Dental pulp
This is loose connective tissue of the tooth, filling the coronal cavity and root canal of the tooth with a large number of nerves and blood vessels; the pulp contains collagen, but no elastic fibers; there are cellular elements represented by odontoblasts, macrophages and fibroblasts. The pulp is a biological barrier that protects the dental cavity and periodontium from infection, and performs a plastic and trophic function. It is characterized by increased activity of redox processes, and therefore high O consumption. Regulation of the energy balance of the pulp is carried out by coupling oxidation with phosphorylation. A high level of biological processes in the pulp is indicated by the presence of processes such as PPP, synthesis of RNA, proteins, therefore the pulp is rich in enzymes that carry out these processes, but carbohydrate metabolism is especially characteristic of the pulp. There are enzymes of glycolysis, TCA cycle, water-mineral metabolism (alkaline and acid phosphotose), transaminases, aminopeptidases.
As a result of these metabolic processes, many intermediate products are produced that come from the pulp into the hard tissues of the tooth. All this ensures a high level of ...., reactivity and protective mechanisms.
With pathology, the activity of these enzymes increases. With caries, destructive changes occur in odontoblasts, destruction of collagen fibers, hemorrhages appear, enzyme activity changes, and exchange of substances in the pulp changes.
Routes of entry of substances into hard tooth tissues and enamel permeability
The tooth has contact with mixed saliva, on the other hand - .... blood, the composition of the hard tissues of the tooth depends on their composition. The main part of the organic and mineral substances that enter the tooth enamel is contained in saliva. Saliva acts on tooth enamel and causes swelling or shrinkage of collagen barriers. As a result, a change in the permeability of the enamel occurs. Substances of saliva exchange with substances of enamel and the processes of de- and remineralization are based on this. Enamel is a semi-permeable membrane. It is easily permeable to H O, ions (phosphates, bicarbonates, chlorides, fluorides, cations Ca, Mg, K, Na, F, Ag, etc.). They determine the normal composition of tooth enamel. Permeability also depends on other factors: on the chemical structure of the substance and the strength of the ion. The sizes of apatites are from 0.13 - 0.20 nm, the distance between them is 0.25 nm. Any ions must penetrate the enamel, but determine the permeability with t.zr. Mr or ion sizes are not possible; there are other properties of the ion’s affinity for enamel hydroxyapatite.
The main route of entry of substances into the enamel is simple and facilitated diffusion.
The permeability of enamel depends on:
1) sizes of microspaces, filled. H O in the enamel structure
2) the size of the ion or the size of the molecule of the substance
3) the ability of these ions or molecules to bind to enamel components.
For example, the F ion (0.13 nm) easily penetrates the enamel and binds to the enamel elements in the damaged enamel layer, therefore it does not penetrate into the deeper layers. Ca (0.18 nm) – is adsorbed on the surface of enamel crystals, and also easily enters the crystal lattice, so Ca is deposited both in the surface layer and diffuses inside. J easily penetrate into the microspace of the enamel, but are not able to bind to HAP crystals, enter dentin, pulp, then into the blood and are deposited in the thyroid gland and adrenal glands.
The permeability of enamel decreases under the influence of chemicals. Factors: KCl, KNO, fluoride compounds. F interacts with HAp crystals, creating a barrier to the deep penetration of many ions and substances. The properties of the pron depend on the composition of the mixed saliva. Thus, secret saliva has different effects on the permeability of enamel. This is associated with the action of enzymes found in saliva. For example, hyaluronidosis > permeability of Ca and glycine, especially in the area of the carious spot. Chemotrypsin and whole phosphatose < CaF and lysine permeability. Acid phosphatosis > permeability to all ions and substances.
It has been proven that amino acids (lysine, glycine), glucose, fructose, galactose, urea, nicotinamide, vit, and hormones penetrate into tooth enamel.
Permeability depends on the age of the person: the greatest - after tooth eruption, it decreases by the time the tooth tissues mature and continues to decrease with age. From 25 to 28 years > resistance to caries, a complex exchange occurs while maintaining a constant composition of the enamel.
Saliva pH, as well as a decrease in pH under dental plaque, where organic acids are formed, permeability increases due to the activation of enamel demineralization by acids.
Caries > permeability. At the stage of white and pigmented spots > permeability, > the possibility of penetration of various ions and substances, as well as Ca and phosphates - these are compensatory reactions in response to active demineralization. Not every carious spot turns into a carious cavity; caries develops over a very long time.
Hyposalivation leads to the destruction of enamel. Caries that occurs at night is a nocturnal disease.
Surface formations on teeth
These are mucin, cuticle, pelicula, plaque, stone.
Mucin is a complex protein, related to salivary glycoproteins, which covers the surface of the tooth and performs a protective function, protects against mechanical and chemical influences, its protective role is explained by the characteristics, specificity of the amino acid composition and the characteristics of the content of sulfur, trianine, which contain up to 200 amino acids, pro... It is attached to sulfur and trianine residues through an O-glycosidic bond. N-acetylneuramine residues. to-you, N-acetylglucosamine, galactose and f..zy. The structure of the protein resembles a comb, which has ... proteins, residues consisting of amino acids, and carbohydrate components are arranged in protein chains, they are connected to each other by disulfide bridges and form large molecules capable of holding H O. They form a gel.
Pellicle
This is a thin, transparent film of carbohydrate-protein nature. Includes glycine, glycoproteins, some amino acids (ala, glu), Jg, A, G, M, amino sugars, which are formed as a result of the vital activity of bacteria. The structure contains 3 layers: 2 on the surface of the enamel, and the third in the surface layer of the enamel. The pellicle covers the dental plaque.
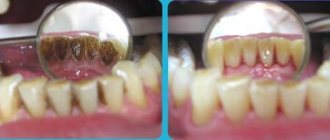
Plaque
White soft film located in the cervical area and on the entire surface. Removed during cleaning and hard food. This is a cariogenic factor. Represents a destructive organ with a large number of substances that are present in the oral cavity, as well as their waste products. 1 g of dental plaque contains 500 x 10 microbial cells (streptococci). There are early plaque (during the first day) and mature plaque (from 3 to 7 days).
3 hypotheses for plaque formation
1) …
2) precipitation of salivary glycoproteins that react in bacteria
3) pricipitation of intracellular polysaccharides. They are formed by streptococci, called dextran and levan. If you centrifuge dental plaque and pass it through a filter, two fractions are separated, cellular and acellular. Cellular – epithelial cells, streptococci, (15%). ....you, diphtheroids, staphylococci, yeast-like fungi - 75%.
In dental plaque, 20% is dry matter, 80% is H O. Dry matter contains minerals, proteins, carbohydrates, and lipids. From mineral ingredients: Ca – 5 mcg/per 1 g of dry plaque. P – 8.3, Na – 1.3, K – 4.2. There are microelements Ca, Str, Fe, Mg, F, Se. F soda in dental plaque in three forms:
1) CaF - Ca fluoride
1) CF protein complex
2) F in M/O structure
Some microelements reduce the susceptibility of teeth to caries F, Mg, others reduce resistance to caries - Se, Si. Proteins from dry plaque – 80%. The protein and amino acid composition is not identical to that of mixed saliva. As amino acids mature, they change. Gly, arg, lys, >glutomate disappears. Carbohydrates 14% - fructose, glucose, hexosamines, salic acids and acids, and glucosamines.
With the participation of enzymes from plaque bacteria, polymers are synthesized from glucose - dextran, and from fructose - levan. They form the basis of the organic matrix of dental plaque. The microorganisms involved in the pre...tion are split, respectively, by dext...heat and levanous cariogenic bacteria streptococci. Available in limited quantities: maktak, pyruvate, acetic acid, propionic acid, citric acid. This leads to a decrease in pH under dental plaque on the surface of the enamel to 4.0. These are cariogenic conditions. Therefore, dental plaque is one of the important etiological and pathogenic links in the development of caries and periodontal diseases.
Lipids
Early dental plaque contains triglycerides, glycerol, and glycerophospholipids. In a mature quantity < , complexes with carbohydrates are formed - glycerophospholipids.
Many hydrolytic and proteolytic enzymes. They act on the organic enamel matrix, destroying it. Relative glycosidoses. their activity is 10 times higher than in saliva. Acidic, alkaline phosphatases, pH, DN-noses. Peroxidases.
The metabolism of dental plaque depends on the nature of the microflora. If streptococci predominate in it, then pH<, but the pH of dental plaque can also increase due to the predominance of actives and staphylococci, which have urealytic activity, break down urea, NH, and deaminate amino acids. The resulting NH combines with the phosphates and carbonates of Ca and Mg and first amorphous carbonate and phosphate of Ca and Mg, non-crystalline HAP - -> crystalline, are formed.
Dental plaque mineralizes and turns into tartar. Especially with age, with certain types of pathology in children - tartar deposits are associated with congenital heart lesions, S.D.
Tartar (ZK)
This is a pathological discalcified formation on the surface of the teeth. There are supragingival, subgingival z.k. They differ in location, chemical composition and chemistry of formation.
Chemical composition of g.c.
Min. content 70 – 90% dry content.
Amount of mineral substances in s.c. various. Dark z.k. contains more minerals than light. What > zk is mineralized, mem > Mg, Si, Str, Al, Pb. First, the low-mineralized substances of ZK are collected, which are 50% composed of bruslit substances Ca NPO x 2H O.
Octocalcium phosphate CaH (PO) x 5H O
Carbonate apatites Ca (PO CO)
Ca (PO) CO (OH).
Hydroxyapatite Ca (PO) (OH
Viktolit – (Ca Mg) (PO)
Is in zk –F is contained in the same z-forms as in dental plaque.
Proteins, depending on the maturity of the cell, range from 0.1 to 2.5%. Number of proteins < as mineralization progresses. In the supragingival zone the soda content is 2.5%. In the dark supragingival zone – 0.5%, in the subgingival zone – 0.1%
Knowledge B. VZK are calcium-precipitating glyco- and phosphoproteins. The carbohydrate part of which is represented by galactose, fructose, ointment. In a ratio of 6:3:1.
The peculiarity of the amino acid composition is that there are no cyclic amino acids
GPL lipids are synthesized by dental plaque microorganisms. Capable of binding Ca to proteins and initiating the formation of HAP. There is ATP in the cell, it is both a source of energy and also a donor of organophosphorus. during the mineralization of brulite and its transformation into TAP. Brulite turns into octocalcium phosphate -> HAP (at pH>8). Brulite - ATP -> octocalcium phosphate -> HAP.
Biochemical changes in hard dental tissues during caries, prevention of caries by remineralization method
Initial biochemical changes occur at the boundary between the surface of the enamel and the base of the tartar. The primary clinical manifestation is the appearance of a carious spot (white or pigmented). In this area of enamel, demineralization processes first occur, especially pronounced in the subsurface layer of enamel, and then changes occur in the organic matrix, which leads to enamel permeability. Demineralization occurs only in the area of the carious spot and it is associated with an increase in the microspace between the HAP crystals, > the solubility of enamel in an acidic environment, 2 types of reactions are possible depending on the acidity:
Ca(PO)(OH) + 8H = 10Ca + 6 HPO + 2 HO
Ca(PO) (OH) + 2H = Ca(HO) (PO) (OH) + CA
Reaction No. 2 leads to the formation of apatite in the structure of which there are instead of 10.9 Ca atoms, i.e. < Ca/P ratio, which leads to the destruction of HAP crystals, i.e. to demineralization. You can stimulate the first type of reaction and inhibit demineralization. Stage 2 of caries development – the appearance of a caries plaque. This is a gel-like substance of carbohydrate-protein nature; microorganisms, carbohydrates, enzymes and toxins accumulate in it. The plaque is porous, carbohydrates easily penetrate through it. 3 floor – formation of organic acids from carbohydrates due to the action of enzymes of cariogenic bacteria. The pH shifts to the acidic side, destruction of enamel and dentin occurs, and the formation of a carious cavity.
Prevention and treatment of caries with remineralizing agents
Remineralization is a partial change or complete restoration of the mineral components of tooth enamel due to components of saliva or remineralizing solutions. Remineralization is based on the adsorption of minerals into carious areas. The criterion for the effectiveness of remineralizing solutions is such properties of enamel as permeability and solubility, disappearance or reduction of carious spots, < increase in caries. These functions are performed by saliva. Remineralizing solutions are used containing Ca, P, in the same proportions and quantities as in saliva, all the necessary microelements.
Remineralizing solutions have a greater effect than mixed saliva.
In saliva, Ca and P combine with organic complexes of saliva and the content of these complexes decreases in saliva. These solutions must contain F in the required amount, since it affects the rejuvenation of Ca and P in the hard tissues of the tooth and bone. At < concentration, HAP precipitates from saliva; in the absence of F, HAP does not precipitate, and octocalcium phosphate is formed instead of HAP. When there is a lot of F, instead of HAP, mineral substances that are unusual for these tissues and, more often, CaF.
Hypothesis of caries pathogenesis
There are several hypotheses:
1) neurotrophic caries is considered as a result of human conditions and the impact of environmental factors on him. The authors attached great importance to the central nervous system
2) trophic. The mechanism of caries development is a violation of the trophic role of odontoblasts
3) appeal theory. Caries is the result of pelation of enamel by complexes of mixed saliva. Caries is the result of simultaneous proteolysis of organs and mineralization of enamel
4) acidogenic or chemical-karyositotic. It is based on the effect of acid-reacting substances on tooth enamel and the participation of microorganisms in the carious process. It was proposed 80 years ago and forms the basis of the modern hypothesis of the pathogenesis of caries. Caries of decalcified tissues caused by acids, image. as a result of the action of microorganisms on carbohydrates.
Cariogenic factors are divided into general and local factors.
General:
include poor nutrition: excess carbohydrates, lack of Ca and P, deficiency of microelements, vitamins, proteins, etc.
Diseases and changes in the functional state of organs and tissues. Adverse effects during teething and maturation and in the first year after teething.
Electrical air (ionizing radiation, stress), which affects the salivary glands, the secreted saliva does not correspond to the normal composition, and it affects the teeth.
Local factors:
1) plaque and bacteria
2) changes in the composition and pH of mixed saliva (pH shift to the acidic side, lack of F, decrease in the amount and ratio of Ca and P, etc.)
3) carbohydrate diet, carbohydrate food residues.
Anti-cariogenic factors and dental caries resistance
1) susceptibility to caries depends on the type of mineralization of hard dental tissues. Yellow enamel is more caries-resistant. With age, the crystal lattice becomes denser and the caries resistance of teeth increases.
2) Caries resistance is promoted by the replacement of HAP with fluorapatites - stronger, more acid-resistant and poorly soluble. F is an anti-cariogenic factor
3) Caries resistance of the surface layer of enamel is explained by the increased content of microelements in it: stanium, Zn, Fe, Va, tungsten, etc., and Se, Si, Cd, Mg are cariogenic
4) Dental caries resistance is promoted by vit. D, C, A, B, etc.
5) Mixed saliva has anti-cariesogenic properties, i.e. its composition and properties.
6) Particular importance is attached to citric acid and citrate.
F and strontium
F is found in all tissues of the body. Available in several forms:
1) crystal. form of fluorapatite: teeth, bones
2) in combination with organic. in-you glycoproteins. Image of an organic matrix of enamel, dentin, bones
3) 2/3 of the total amount of F is in the ionic state in biol.
liquids: blood, saliva. A decrease in F in enamel and dentin is associated with a change in pit.H O.
It is easier for F to be included in the enamel structure in a slightly acidic environment, the amount of F in bones increases with age, and in the teeth of children it is found in increased quantities during the maturation of hard tooth tissues and immediately after eruption.
With very large amounts of F in the body, poisoning with fluorine compounds occurs. It is expressed in increased fragility of bones and their deformation due to disruption of R-Ca metabolism. As with rickets, but the use of vitamin D and A does not cause a significant effect on the disturbance of P-Ca metabolism.
A large amount of F has a toxic effect on the entire body, due to a pronounced inhibitory effect on the metabolic processes of carbohydrates, fats, and tissue respiration.
Role F
They take part in the process of mineralization of teeth and bones. The strength of fluorapatites is explained by:
1) amplification bonds between Ca ions in the crystal lattice
2) F binds to organic matrix proteins
3) F contributes to the formation of more durable crystals of HAP and F-apatites
4) F helps to activate the process of precipitation of apatites of mixed saliva and thereby increase. its remineralizing function
5) F affects the bacteria of the oral cavity, acid-forming properties are burned and thereby prevents the pH from shifting to the acidic side, because F inhibits ecolase and suppresses cliquelysis. The anti-caries effect of F. is based on this mechanism.
6) F takes part in regulating the entry of Ca into the hard tissues of the tooth, reducing the permeability of enamel to other substrates and increasing caries resistance.
7) F stimulates reparative processes in case of bone fractures.
 F reduces the content of radioactive strontium in the bones and teeth and reduces the severity of rickets. Sr competes with Ca for inclusion in the HAP crystal lattice, and F suppresses this competition.
F reduces the content of radioactive strontium in the bones and teeth and reduces the severity of rickets. Sr competes with Ca for inclusion in the HAP crystal lattice, and F suppresses this competition.
Ascorbic acid. Function. Role in the metabolism of tissues and organs of the oral cavity
1) the effect of the vitamin is associated with its participation in OM reactions. It accelerates the dehydrogenation of reduction. coenzymes NADH, etc., activates the oxidation of glucose by PFP, which is so characteristic of dental pulp.
2) Vitamin C affects the synthesis of glycogen, which is used in teeth as the main source of energy during the mineralization process.
3) Vit.C active. many enzymes of carbohydrate metabolism: in glycolysis - hexose, phosphofructokinose. In CGC...hydrogenosis. In tissue respiration - cytochrome oxidosis, as well as mineralization enzymes - alkaline phosphatosis
4) Vit.C is directly involved in the biosynthesis of protein, compound, procollagen in its transformation into collagen. This process is based on 2 reactions
proline - -axiproline
Ph-t: proline hydroxylase, cof-t: vit C.
Lysine – oxylysine f-t: lysine hydroxylase, cof-t: vit.C
Vitamin C performs another function: activation of enzymes by reducing disulfide bridges in enzyme proteins to sulhydryl groups. As a result of activation of alkaline phosphatosis, ... dehydrogenase, cytochromexidosis.
Vitamin C deficiency affects the condition of the periodontium, the formation of intercellular substance in the connective tissue decreases
5) vitamin deficiency changes the reactivity of tooth tissue. May cause scurvy.
What is included in the structure: basic elements
The structure of the tooth is complex, it includes not only the enamel. Dentists divide this layer into three components that are connected to each other:
- Prism. Consists of one ameloblast cell. These elements are a kind of foundation of the enamel, running across its entire thickness perpendicular to the connection of the protective layer with dentin. According to their shape, prisms are divided into oval, arched and polygonal; their thickness ranges from 3 to 5 microns.
- Interprismatic substance. This is a kind of binding component that tightly envelops all enamel prisms. The level of mineralization of this substance is slightly lower than that of prisms, and its thickness cannot exceed one micron. The spindles are located in the interprismatic space. These are a kind of processes of odontoblasts, the bodies of which are enclosed in the nucleus pulposus. The spindles grow from the area where the pulp is located, penetrating the enamel and reaching its surface. The processes are responsible for the ability to recognize toothache.
- The cuticle is the most important component of the composition; it is the surface shell of the enamel, which, after eruption, is erased in the area of their chewing surface and is partially preserved in the lateral areas.
Many studies have been devoted to the characteristics of the chemical composition of tooth enamel. Scientists have studied this component in detail in order to create new, more effective methods of dental treatment. Knowing exactly the composition of enamel, it is possible to develop highly effective means for strengthening and restoring it.
In addition to the main components of the enamel layers, it also contains others:
- Neonatal line - visually looks like a dark stripe, present only on milk units. This strip is located in places where two types of enamel intersect: the layer formed before the birth of the child, and the layer that formed immediately after the baby was born.
- Bundles and plates are specific formations that contain hypomineralized prisms; between them there is an interprismatic substance consisting of the same material as the prisms themselves. Chemical analysis demonstrates that, in terms of molecular structure, this material contains a huge amount of protein compounds. Many dentists unanimously claim that it is through the tufts that various bacteria enter the inner layers of enamel from the oral cavity. Then they make their way further, getting into the layers located inside the tooth, thereby causing caries.
- Gunter-Schräger stripes are stripes that stand out in shades on the enamel surface. The shade can be light or dark. Located in a perpendicular position relative to the surface of the enamel, they are formed as a result of opening the prisms.
- Retzius lines are shaped like slightly offset arches, located symmetrically to each other.
Specifics of enamel of baby teeth
The main feature of the enamel of the first dental units is that this layer is not as strong as that of permanent teeth. This can be justified by the fact that much fewer mineral compounds are concentrated in baby teeth. If you study the composition of children's enamel in detail and examine it under a microscope, you can draw the following conclusions:
- The Retzius lines, which we mentioned above, are not as pronounced as on constant units.
- The prisms are placed in a horizontal direction.
- There are many microscopic cracks in the enamel of a baby tooth, hence the porosity of its structure.
Given this information, pediatric dentists claim that children's enamel is more susceptible to wear and destruction. For this reason, caries progresses at an intensive rate in children.
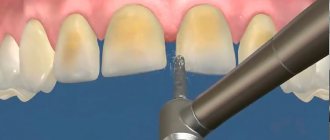
Dental enamel implantation
The newest technique for restoring tooth enamel is its implantation. Thanks to this method, the patient’s teeth change shape, their color and the bite is corrected. Restoring enamel for front teeth is especially effective, when restoring their aesthetic appearance is one of the main tasks.
The connection between tooth enamel and implant occurs at the molecular level, and the special composition of the substance, almost identical to natural tooth enamel, allows you to achieve the greatest effect. Artificial enamel can provide the tooth with maximum protection, which is why this method is becoming increasingly popular and approved by dentists.
Damage to enamel: what to do
One of the most common phenomena is damage to the enamel. There are several types of lesions. It is worth considering that all types of lesions can be classified into two groups: those that formed before the eruption of baby teeth, and those that arose after. The first category includes the following pathologies:
- Dysplasia – there are a lot of symptoms of the disease: the formation of spots, thinning of the enamel layer. Most often, this pathology is associated with genetics and is often accompanied by problems with bone development. Teeth affected by dysplasia erupt with an initially irregular shape.
- Hypoplasia. Tooth tissues atrophy or are absent altogether. The formation of spots, changes in shade, thinning of the enamel are the main signs of the disease. To treat this problem, medications are prescribed that help restore mineral balance.
- Hyperplasia is excess tissue growth. It can also be caused by a mineral imbalance. Various blood diseases and hormonal imbalances can lead to the development of hyperplasia of the tooth buds. Enamel “drops” appear on the tooth surface. In rare, particularly difficult cases, hypertrophied areas can be filled with dentin or pulp.
- Fluorosis. The disease is characterized by the appearance of spots and furrows. Small pits or streaks may appear. Pathology is caused by excess fluoride content in the body. Often the disease affects baby teeth.
Below we will consider pathologies that are not associated with genetics and congenital anomalies:
- Erosion refers to non-carious lesions of the enamel layer and dentin. Erosion can be triggered by gastrointestinal diseases, excessive consumption of too acidic foods, and taking certain types of medications. Drug treatment is prescribed.
- Hypersensitivity. When a person reacts painfully to too cold or hot, this indicates the development of tooth sensitivity, which is often accompanied by thinning of the enamel. In this case, the task is to strengthen the layer. Minerals and vitamins are prescribed.
- Wedge-shaped defect. The pathology is that the neck of the tooth is gradually exposed, and the base is destroyed. The disease can be triggered by thyroid disease, gastrointestinal problems, and gum disease.
- Caries. If the carious lesion of the enamel layer is not eliminated in a timely manner, the disease will spread to the dentin and soon reach the pulp. The disease is treated by cleaning the cavity and installing a filling there.
- Impacts of a mechanical nature. Chips and cracks often form on the enamel. Modern dentists, using advanced equipment, can restore the damaged area.
Types of damage to tooth enamel before teething
Dysplasia
A number of disorders, which is characterized by a number of signs: gray spots, thinning of the enamel or the absence of fragments of the enamel layer. In the vast majority of cases, dysplasia is associated with genetic abnormalities, metabolic disorders and bone diseases. When teething, teeth may be irregular in shape (triangular, pear-shaped, etc.).
To treat erosion of tooth enamel, multivitamins, sodium fluoride, and electrophoresis are prescribed. In case of extensive lesions, artistic restoration and prosthetics are used to restore aesthetics. To avoid complications, dental enamel dysplasia in children requires immediate treatment.
Hypoplasia
With hypoplasia, atrophy of tooth tissue or its complete absence is observed at the intrauterine level. Usually the disease is associated with a mineral imbalance. Hypoplasia is expressed by a change in color (to gray or brown), the appearance of spots, thinning of the enamel and even its complete absence (aplasia).
When treating thinning enamel, medications are prescribed to restore mineral balance (calcium gluconate solution, etc.), as well as a complex of vitamins. In case of aesthetic problems, whitening can be performed, and in severe cases, the tooth is covered with a crown or veneer.
Hyperplasia
Excess dental tissue, the appearance of which is also caused by an imbalance of mineral balance (usually due to hormonal imbalances in parents or blood diseases). On the surface of the tooth, so-called enamel drops are formed - islands of brown or reddish color. For more complex anomalies, hypertrophied areas can be filled with dentin or pulp.
Treatment of hyperplasia includes polishing teeth with a drill, applications and rinsing with fluoride and calcium solutions in combination with taking medications that normalize the mineral composition and eliminate the root cause of the deviation.
Fluorosis
When dental fluorosis occurs, spots, grooves, pits, or streaks form on the surface of the enamel. The disease is caused by an excess of fluoride in the body and often occurs in children.
Remineralization of enamel, grinding of teeth, and normalization of the amount of fluoride in the body are indicated. In difficult cases - orthopedic treatment and artistic restoration.
Changes in the structure and violation of the integrity of the enamel can be caused by a number of genetic abnormalities and hereditary diseases. That is why it is important to establish the root cause so that the best treatment can be prescribed if tooth enamel is damaged or atrophied.
Methods of strengthening
To eliminate pathologies associated with tooth enamel, dentists use various techniques, both therapeutic and orthopedic. For severe lesions, crowns or veneers are used. But the best option for every person is to try to preserve their own healthy teeth. What options for strengthening enamel are suitable for an adult:
- Take vitamins and minerals regularly.
- Use preventive and therapeutic agents – gels and pastes containing calcium and fluoride.
- Regularly visit the dental office for preventive examinations and professional cleanings. This will not only protect the enamel, but also prevent the development of caries.
- If the enamel is damaged and visual appeal is lost, you can undergo a whitening procedure, but only after prior consultation with your doctor.
For children, dentists recommend fluoridation, fissure sealing and other procedures aimed at comprehensively strengthening teeth. Parents should pay attention to their child's diet. It should not contain sweet soda or excessive amounts of sweets. Since tooth enamel contains a large number of microelements, it is worth taking vitamins periodically, after consulting with a pediatrician and pediatric dentist.
Strengthening the enamel of molars
There are more methods to preserve molars and maintain the condition of their enamel layer. Firstly, this is due to fewer contraindications for adults. Secondly, molars require long-term strengthening.
The main methods of strengthening the enamel of permanent teeth include:
- Drug therapy is based on the use of vitamin complexes containing vitamins of groups B6, B12, D. In addition, the patient is selected drugs that promote better absorption of calcium and fluoride by the body.
- Special gels and oral hygiene products – this technique uses specialized toothpastes and gels containing components necessary for teeth to strengthen and maintain the condition of the enamel layer. Also, teeth are subjected to unimaginative cleaning in a dental office.
- Mineralization and preventive cleaning – mineralization is performed using special means to increase the strength of the enamel and reduce its susceptibility to a number of negative factors. As for cleaning, such procedures are performed by dentists in the clinic using special equipment. During cleaning, plaque and tartar are eliminated , pathogenic bacteria and microorganisms that can harm the enamel layer are removed.
- Home prevention - to maintain healthy teeth and enamel, patients are advised to perform a light massage of the gums, enrich the diet with fresh vegetables and fruits rich in vitamins.
Author: Zhukov M.A.