About caries and the fight against it with fluoride
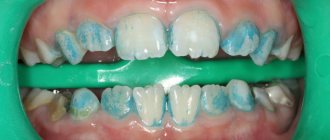
There are three main factors that influence the development of dental caries: tissue susceptibility, the presence of an infectious strain of bacteria (Streptococcus mutans), excess sugar and other nutrients that stimulate bacterial growth. As pathogenic bacteria grow, an acidic byproduct is produced that can dissolve the minerals in the enamel. As a result, the tooth is destroyed.
Dentists recommend the use of fluoride solutions for treating the oral cavity and drinking fluoridated water. It is thanks to fluoride that there is an improvement in the condition of teeth and a sharp reduction in caries. Similar improvements have been recorded in different studies and in almost all age groups. However, tooth decay remains the most common infectious disease, and fluoride treatment remains an important tool in the fight against dental and oral diseases.
Applications of sodium fluoride
The use of sodium fluoride is not limited to any particular industry. The use of the chemical applies to:
- Production of medicines and toothpaste. Sodium fluoride in toothpaste and preparations is used to treat and prevent bone diseases.
- Production of aluminum in metallurgy, where the substance is used to clean metals and create an anti-corrosion coating for parts.
- Wood processing where sodium fluoride is used as an antiseptic and wood preservative.
- Agriculture, where the substance is used to control harmful insects.
- Water treatment for enriching water with fluorine.
- The area of production of glass and ceramic products, enamel, refractories, fluxes.
- Laboratory research in which a substance is used as a reagent.
- The pharmaceutical industry, in which the substance is used to test drugs.
- Biochemical industry in which sodium fluoride is used to preserve tissue samples.
- The sphere of gas purification from uranium fluorides.
Also, the use of sodium fluoride extends to the chemical industry, where the reagent is used for various reactions, including the synthesis of organofluorine compounds, reagents, freons and others.
What is fluoride and how does it work?
Fluoride is a form of elemental fluorine, which is normally a toxic gas. Fluoride's chemical reactions with other compounds, such as tin, give it unique properties that increase the element's effectiveness in treating the oral cavity.
Once in the mouth, fluoride is diluted by saliva, depositing as a bacterial plaque on the surface of the teeth. In this state, it directly inhibits the growth of bacteria, so less acid is produced in the mouth. The fluoride stored in the plaque is then released when the bacteria produce enough acid to reduce the acid-base balance in the mouth. When this happens, fluoride diffuses into the tooth through tiny pores in the enamel. Fluoride ions replace the hydroxyl ions in the hydroxyapatite crystals that make up tooth enamel and form a new compound called fluorapatite. This compound is almost insoluble in the acids produced by bacteria in the mouth, so it helps protect teeth from decay.
What is fluoride water?
The presence of fluoride ions in water can be absolutely safe and deadly. In microscopic doses, F(-) fluoride ions have a therapeutic effect, but in large concentrations they can kill. The amount of fluoride in a regular tube of toothpaste is such that it could kill a small child if he ingested the paste. A dose of 2-3 grams of sodium fluoride is lethal for an adult.
Water with fluoride ions is much more toxic than lead. In many countries, aqueous solutions are specially fluoridated, since this microelement is necessary for the formation and normal development of dental and bone tissue in the body. The most popular method of fluoridation is the addition of sodium fluoride. This is the most expensive reagent used in public utilities to enrich tap drinking water with fluoride F(-).
When fluoridating it is important:
- strict adherence to technology,
- exact dosage,
- regular supply of fluorine-containing raw materials to water treatment facilities.
Due to the strong toxicity of the chemical element, excessively high fluorine content in water in quantities above established standards is considered dangerous. Many countries - China, Japan, India, Israel - not only refused, but also introduced a ban on fluoridation of drinking water.
From the history of fluoride
Frederick S. McKay, a dentist practicing in Colorado Springs in the early 1900s, was the first to discover that fluoride was an effective killer of oral infections. McKay noticed that many of his patients had mottled enamel or brown spots on their teeth. By 1916, Mackay and his associates discovered that the spotting was caused by something in the patients' drinking water. It took him another 12 years to understand how this effect was related to the absence of dental caries in patients. And another three years to understand the chemical mechanism and nature of the phenomenon. In 1931, Mackay realized and became convinced that patients with stained teeth were drinking unusual drinking water. She had unusually high levels of natural fluoride.
The chemical composition of water and the mechanism by which fluoride affects human tooth enamel were studied in detail by scientists during the 1930s and 1940s. Careful testing of the water led to the conclusion that one part of fluoride per million parts of water was the ideal level. This concentration significantly reduced the risk of dental caries without causing stains on the enamel.
This research led to government water fluoridation programs, for example, in America. In Russia today, some cities are also equipped with systems where water is treated with fluoride. The idea of using fluoride in oral care products dates back to 1956. There are now hundreds of fluoride-containing products available to the public, as well as to dental professionals.
How do fluorides work?
A smile says a lot, but its absence can mean even more. People are often reluctant to smile if they are not confident in the beauty of their teeth. Most of us understand why we need to brush and floss, and that's a good thing. But mechanical teeth cleaning alone is not always enough to keep teeth healthy. Using fluoride products is an important part of dental care. After reading this article, you will know the answer to the question: How does fluoride improve your dental health?
What are fluorides and how do they work?
What is fluoride?
If you thought that fluorides were synthetic additives in oral hygiene products, you were not alone in your misconceptions. In fact, fluorides are natural compounds: they form naturally in the earth's crust and are widely present in nature. Fluorides are found in a number of foods and also in water. In many countries, drinking water with low levels of these substances is additionally fluoridated to help consumers strengthen their teeth and protect against caries.
Fluoride's ability to prevent tooth decay was discovered in the 1930s when researchers discovered that children who drank naturally fluoridated water suffered less tooth decay than those who drank fluoride-free water. Since then, studies have shown time and time again that where drinking water is fluoridated, the incidence of dental caries decreases.
The World Health Organization, the International Dental Federation and many other medical societies support and promote the idea of fluoridation of drinking water to combat tooth decay. According to experts from the Russian Dental Association, the unique properties of fluoride allow it to be considered the most effective means of preventing dental caries in children and adults.
How do fluorides work?
To understand the mechanism of action of fluorides, you need to study the structure and functioning of enamel. The processes of demineralization and remineralization constantly occur in it. The reason for the loss of minerals from the enamel structure is the activity of bacteria that live in the oral cavity. By feeding on sugar and other carbohydrates, these microorganisms secrete acids that dissolve tooth enamel. Fluorides protect enamel from damage caused by demineralization and make hard tooth tissue less susceptible to the negative effects of acids.
At other times, after neutralizing the “acid attack,” fluorides help the enamel replenish the loss of calcium and phosphate ions, which give teeth greater hardness. This process is called remineralization. Excessive loss of minerals and insufficient replenishment of them in the enamel structure lead to the formation of caries.
What are the benefits of fluoride?
Fluoride helps protect teeth in two ways. When children receive adequate daily doses of fluoride in food or water, these compounds enter the bloodstream and participate in the process of tooth formation, that is, they act from the inside. In addition, fluorides remain in saliva and help strengthen the teeth on the outside, so that acids cause less damage to the enamel. Thanks to the effects of fluoride, teeth remain strong and resistant to caries, and there is no increased sensitivity.
Fluoride Source:
Fluorine
What is fluoride?
Now it's time to remember chemistry. Fluorine is a chemical element that, as noted by the Great Encyclopedia of Oil and Gas, is highly reactive. Thanks to this property, fluorine easily forms compounds with other substances. Some of the best known fluorine compounds found in nature are fluorides. This means that the fluoride contained in toothpastes is a natural ingredient.
Examples of these compounds include calcium fluoride, sodium fluoride, sodium monofluorophosphate and tin fluoride. These salts are often used in the manufacture of toothpastes.
How does fluoride strengthen teeth?
When you use a fluoride-containing oral hygiene product, such as toothpaste, the source of fluoride enters your saliva. Saliva washes over the teeth and fluoride is absorbed onto the surface of the enamel. There it binds with the calcium and phosphate present in the enamel structure to form fluorapatite, a hard mineral that can resist tooth damage and help prevent the formation of cavities.
Is fluoride safe?
Fluorides are safe and effective when used correctly in the proper amounts, but the rule of “everything in moderation” applies to them as well. Long-term studies have shown that the main danger associated with excessive consumption of fluoride is fluorosis, a disease that can develop in a child if, at an early age, during the formation of teeth, he ingested water or foods with a high content of fluoride compounds for a long time.
In this case, white and sometimes even brown and gray stripes and spots may appear on the child's teeth. In addition to the high fluoride content in drinking water, above the established sanitary standards, there is another danger of excessive systemic intake of fluoride into the child’s body - when he uncontrollably swallows too much fluoride-containing toothpaste. This is why it is important to supervise children while brushing their teeth and make sure they always spit out the toothpaste. Additionally, it is best to keep sodium fluoride tablets out of the reach of small children. If you have questions or doubts, discuss them with your dentist: he will tell you how best to act to protect your child not only from tooth decay, but also from fluorosis.
Why is fluoride safe?
The effectiveness of fluorides in reducing the prevalence of dental caries is widely documented in domestic and foreign literature and is an indisputable fact. Rigorous evidence from numerous studies, WHO World Health Assembly resolutions, the position of the International Dental Federation (FDI) and the International Association for Dental Research (IADR) confirm the medical and economic effectiveness, as well as the safety of daily use of fluoride in optimal amounts for prevention. dental caries. There is no scientific evidence that consuming fluoride in adequate amounts is associated with adverse health effects. On the contrary, evidence is presented that the amounts of fluoride contained in toothpaste produced in accordance with the interstate standard and tap drinking water that meets established sanitary standards are both safe and effective.
Dental fluoridation and fluoride supplements
Means for fluoridation of teeth
There are several ways to fluoride teeth. Topicals are applied directly to the teeth: these include toothpastes, mouth rinses and preparations for professional dental fluoridation in the clinic. Although the interaction time of such fluoride-containing products with the surface of the teeth is short, they are capable of creating a so-called fluoride depot in the oral cavity, which will ensure the constant presence of fluoride ion in the mouth for several hours.
If you have generally healthy teeth, drinking fluoridated water and using fluoride toothpaste is usually sufficient to effectively prevent tooth decay. If your area's tap water is not fluoridated or you drink bottled water without fluoride, your dentist may recommend having your teeth professionally fluoridated to properly protect them.
Products for this procedure are available in the form of gels, foams and varnishes. They contain higher levels of fluoride than toothpastes and rinses, so they are intended for use in dental clinics only.
Fluoride Supplements
If you are concerned that your child is not getting enough fluoride, ask your pediatrician or dentist for a prescription for a fluoride supplement. These supplements are suitable for children aged 6 months to 16 years, as long as they do not drink fluoridated water. Fluoride preparations are available in the form of drops for young children and tablets for older children and adolescents.
If a child needs more fluoride than is provided in the supplement, the dentist or pediatrician may recommend using an additional fluoride mouthwash or gel. In any case, be sure to supervise your child when using any product containing fluoride, and keep dietary supplements out of the reach of children.
Like any other path, the path to healthy teeth and gums begins with the first step. This includes good oral hygiene: brush your teeth with fluoride toothpaste at least twice a day and floss daily to remove plaque from between your teeth. And, of course, don’t forget to visit your dentist regularly.
Is fluoride dangerous?
Despite the fact that fluoride has been actively used by humanity over the past decades, there are still concerns about the health of people about the consequences of its use. Researchers believe that high levels of fluoride may slow the natural formation of tooth enamel. The fact is that the abundance of fluoride creates hypomineralization, which negatively affects the condition of the teeth - fluorosis (Latin Fluorum - fluorine + osis). Dentists continue to recommend fluoride products to adults and children. But they warn that it is possible to reduce the risk of fluorosis. Children just shouldn't swallow toothpaste.
Although doctors and scientists claim that fluoride is largely responsible for improving dental health, there are those who claim that it can cause bone cancer. In the 1980s, a study by the National Toxicology Program found "equivocal evidence" of carcinogenicity based on testing done on rats. As a result, it was concluded that there is no reliable evidence that fluoridation of water and hygiene products can lead to the development of cancer.
Fluorine content in various products
There are many fluoride compounds approved for use in oral care products. Ingredients, including fluoride compounds, must be listed on the label. The fluoride content must be appropriate for the type of product. It is expressed as a percentage. For example, toothpaste from a number of manufacturers may have something like this:
- sodium fluoride: 0.22%;
- sodium monofluorophosphate: 0.76%;
- tin fluoride: 0.4%.
Mouth rinses have lower concentrations of fluoride. Typically it is 0.02%. In these products, fluoride is usually mixed with the following components: sodium phosphate, phosphoric acid. The healing gel contains about 0.4% tin fluoride.
In addition to these products, there are many others in hygiene products, such as solvents, thickeners and pH control agents. Solvents include water or glycerin, which are used as a base. Deionized or demineralized water is used to prevent unwanted minerals from affecting product stability. The water concentration in the formula can be 90% or more. Thickeners are added to regulate viscosity. These include xanthan, carrageenan and various other polymers, used in concentrations ranging from 0.1 to 2.0%. Flavors and colors are added to make products more attractive and palatable. Popular flavors include mint, eucalyptus, grape, and strawberry. Dyes are used to impart color. Because the product may be accidentally ingested, these colorants are approved for use in foods. Preservatives are added if necessary. Depending on the pH of the product, they may be required to prevent the growth of mold or bacteria in the product while it is stored on the shelf. One or two tenths of a percent is a typical level of preservative use. Organic acids such as phosphoric acid may be added to control the pH of the product.
Fluoride-containing treatments are designed to provide the appropriate concentration of fluoride at normal pH levels. If fluoride levels are too low, treatment will not be effective. If it is too high, patients may become poisoned. Countries have created laws to ensure that these products are safe and effective. Specialized fluoride-containing preparations are produced not only in the form of solutions, but also in the form of gel and foam. They can be made in the form of solutions that are poured into plastic trays that are attached directly to the teeth. Approximate composition of professional products enriched with fluoride:
- acidified phosphate fluoride gel with 1.23% fluoride ion, intended for pouring into a tray;
- sodium fluoride with 2% fluoride ion at pH 7.0, for use in etching porcelain crowns;
- a liquid solution of tin fluoride with 0.63% fluoride ion, intended to prevent caries, reduce the formation of tartar and plaque, reduce inflammation and bleeding of the gums;
- fluoride with 1.23% fluoride ion at pH 3-4.25 - a foam that heals by saving the patient from ingesting an unwanted substance.
Safety measures during storage and transportation
Whether sodium fluoride will cause harm or benefit depends on how well safety precautions are followed when working with a toxic substance. In production facilities where sodium fluoride is used, air composition is regularly analyzed to assess the MPC content. Employees who interact with toxic chemicals must wear personal protective equipment. The workplace is equipped with a forced ventilation system.
Transportation of sodium fluoride occurs by any means of transport. Warehousing and storage of toxic substances is possible only in dry, well-ventilated areas.
The manufacturing process of products containing fluoride
The manufacturing process for fluoride products is similar to those used to manufacture other oral care formulations. Since most fluoride compounds used in hygiene and treatment are water-soluble, these products are relatively easy to manufacture.
The production process involves simply mixing the ingredients. It does not require the inclusion of any special solvents, reagents or emulsification technology. Large batches of products can be produced in stainless steel tanks. The first step is mixing water with glycerin. This is the basis of most products. Other ingredients are added sequentially. Depending on the type of final product, heating and cooling of the solutions may be required to dissolve the components.
At the end of the process, pH regulators are introduced. They ensure that the product has the correct balance of acid base. Flavorings are added at the end of the operation as they are heat sensitive. Once all processes are completed, the formulation is tested for pH, weight percent solids, and fluoride concentration. Next, the product is bottled into individual bottles with sealed caps. Products packaged in test tubes or with foaming dispensers require more complex sealing mechanisms during packaging.
Fluoride levels in water
Due to the toxicity of the gas, the fluoride content in drinking water is strictly standardized. In 1994, according to WHO standards, a maximum permissible value was established that should not be exceeded: the maximum permissible concentration for fluorine in water is 0.5 mg/l . In Russia, the maximum permissible concentration of fluorine for water is established by GOST 2874-90.
The normalized presence of fluoride - the amount of fluoride in drinking water - depends on the area and climate. Where there is year-round heat, where people drink a lot of liquid, the recommended figure is 0.5 mg/l. In the northern territories, the norm is set at no higher than 1 milligram. In the middle zone in areas with a temperate climate, it is recommended to stick to 0.7 mg/l.
The requirements are not met everywhere. Almost a third of the world's population drinks liquids in which the concentration of fluoride in water is above 1.5 mg/l. It is imperative to check the exact composition of elements and compounds in the aqueous solution and remove excess fluoride in tap or artesian water.
In Indian, South American and Kenyan waters there is a dangerous excess of fluoride - 25 mg per liter. In many southwestern regions of Ukraine, extremely low F(-) levels are observed. In Russian underground deposits, the fluorine content in water is increased, much more than 1.5 mg/l. In the Moscow region, in the Urals it reaches 4.4 ml/l. In water bodies of Kazakhstan and Azerbaijan, the value is critical, up to 11 ml/g.
In surface water sources the concentration is much lower, up to 0.3 mg/l. In particularly hazardous areas, the saturation of aqueous solutions with F(-) ions can exceed 65 mg/liter. Sea salt waters are less dangerous; the presence of fluorides in them is low, only 1.1-1.4 mg.
For areas with a low fluoride content (below the optimal fluoride content in water), health care recommends adding them artificially - fluoridating the aqueous solution before use. Fluorides are added in small quantities: micrograms or even nanograms.
Indications for fluoridation are:
- low maximum permissible concentration of water for fluorine,
- lack of regional programs for food fluoridation,
- medical indicators - a large percentage of caries diseases in this area.
For areas where F(-) ions are present in excess, you need to choose ways to reduce the fluoride content in water.
In addition to the fact that public utilities responsible for water supply are required to monitor the maximum permissible concentration for fluorine in water, each person can independently check the composition of the water and purify it of all harmful substances before use.
How relevant will fluoride products be in the future?
In the future, it is possible that products containing fluorides will have serious competitors. But today they still remain an important tool in the fight against infectious diseases of the teeth and oral cavity. New technological advances are appearing, in particular, for example, British researchers have discovered a new type of anti-caries agent that stops the development of caries in 3 months. The new ingredient is a protein fragment called p1025 peptide. It attaches to the surfaces of teeth and neutralizes pathogenic bacteria. It is likely that such developments and discoveries will one day help create fluoride-free products for the prevention and treatment of caries.
How is water purified from fluorides?
To reduce fluoride levels in drinking water, you can use one of several available methods. The most common are two of them: chemical and electrolyte.
- Chemical cleaning involves the use of certain reagents. Some of the most popular are oxides of magnesium and aluminum. Through this treatment, fluorine and fluoride ions are bound and subsequently removed. This is the cheapest method, which, however, does not guarantee complete purification of drinking water.
- The electrolytic method is used when preliminary cleaning is necessary - with its help you can reduce the level of wear of installed filters, as well as get rid of large contaminants.
Activated carbon filters are also a cheap cleaning option but require frequent replacement to remain effective. This option is most often used for home filtration. To achieve greater productivity, it is recommended to install filters with reverse osmosis - they have a special membrane that prevents the penetration of impurities and organics.
According to experts, membrane filters demonstrate the greatest efficiency at home. If not all water must be filtered, hybrid systems are used that support several stages of purification. It is also allowed to separate water flows for domestic needs, as well as for consumption and cooking.